Anti-PD-1 and anti-PD-L1 immunotherapies are innovative and promising treatments in the fight against certain types of cancer. What is their mode of action? What are their benefits? Here we explain.

Immune system and cancers
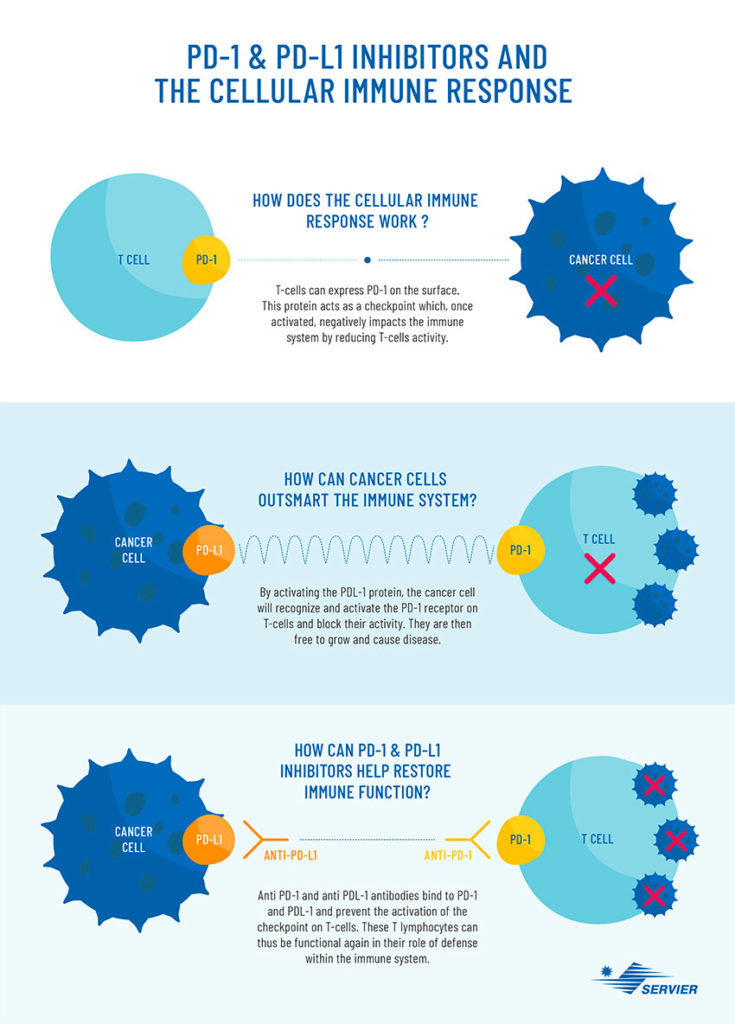
The role of the immune system is to protect the body against infections and diseases. In response to a bacterium, virus, infection, or abnormal cells such as tumor cells, the immune system is activated and lymphocytes such as B cells, which produce antibodies, and T cells can identify and kill abnormal cells.
In the case of cancer, as the disease progresses, the cancer cells cause a genetic modification which helps to hide and become indestructible by the immune cells. Result: the tumor cells produce proteins which inactivate the body’s defences and continue to develop and weaken the body.
Immunotherapies, how do they work?
Unlike chemotherapy which directly targets and destroys cancer cells, immunotherapy seeks to provoke or improve the immune response against these cells. There are different immunotherapy strategies, notably therapeutic vaccines, cell therapies, monoclonal and bispecific antibodies such as anti-PD-1 and anti-PD-L1 antibodies. Most frequently these treatments are indicated for advanced or relapsed metastatic cancer.
ANTI-PD-1 and ANTI-PD-L1 antibodies: What role do they play?
In the early 1990s, the immunologists James Allison, from the University of Texas, and Tasuku Honjo, from the University of Kyoto, were able to determine why the immune system does not respond effectively to cancer cells. Ordinarily, the immune system produces specific proteins known as “check points”, which control the functional activity and killing capacity of immune cells. Among these, PD-1 acts as a receptor on T cells, which binds to PD-L1 expressed on cancer cells, and by this binding the T-cell capacity to kill cancer cells is reduced.
The whole purpose of immunotherapy is to lift the immune brake that the tumor cell exerts on the immune system. In concrete terms, it involves injecting antibodies which will attach to the tumor cell (PD-L1) and T cells (PD-1), this blocks the binding between PD-1 and PD-L1 and consequently allowing the immune system to return to its initial function and fight the disease.
Anti-PD-1 antibodies are especially indicated in the management of melanoma, lung cancer, kidney cancer, and Hodgkin lymphoma (a lymphatic cancer). Anti-PD-L1 antibodies are the preferred treatment for certain cancers of the bladder such as urothelial carcinoma, bronchial cancer, triple negative breast cancer (the most aggressive form of breast cancer), and Merkel cell carcinoma (a rare and aggressive skin cancer). The first marketing authorizations (MA) were granted in 2011 in the United States and in 2013 in Europe and the number of prescriptions has exploded in recent years. In France, the first anti-PD-L1 was granted MA in 2015, administered in the treatment of non-small cell lung cancer.
Efficacy and side effects
They can, however, cause potentially serious and complex adverse reactions, particularly immunologic reactions resembling to auto-immune reactions. These recent treatments are also very costly – between 4 000 € and 12 000 € per treatment, depending on the antibodies – and still require extensive research.
In order to continue research and accelerate the use of these targeted therapies, the fundamental challenge is to select “responsive” patients in whom the treatment will be effective. The results observed are extremely variable; highly effective in some patients but rarely accelerated growth of the tumor in others. Currently, only few patients respond to these protocols. It is therefore essential to identify the patient population, which has the highest chance to respond to these compounds, notably through the study of biomarkers. No truly reliable biomarkers have been clearly determined to date, but researchers have however observed that tumors with a high mutation rate appear to respond better to immunotherapy.
AND SERVIER?
Servier has made oncology one of its foremost priorities, investing more than 50% of its R&D budget in the field. The Group hopes this will help it become a renowned innovator in developing cancer treatments.
The Group’s strategy in oncology is:
– To target hard-to-treat cancers where generally speaking needs are not yet met and hard to treat;
– To concentrate on promising approaches such as immunotherapy;
– To draw on a wide range of expertise by relying on high-level teams that have been strengthened by the acquisitions, and by developing a network of diverse partners.
First generation immune checkpoints blocking antibodies have transformed the treatment of cancer in multiple indications and lines of treatment. Yet only 10-60% of patients will respond across indications and the majority of these responders will relapse outlining a strong medical need beyond PD-1/PD-L1 blocking antibodies.
Over the years, significant strides have been made in immune checkpoint modulation research, revealing several inhibitory and stimulatory receptors, which are now being exploited by Servier for the development of next generation immune checkpoint-based therapies. This effort includes several programs at research- and clinical-stage.

