Clinical trials refer to the series of scientific studies carried out in humans that validate the research and development stages in the medicine discovery process.
They are a crucial step, and subject to rigorous regulatory controls, in obtaining a marketing authorization and determining the optimal use of a medicinal product to ensure therapeutic benefit for patients.
Clinical trials can last several years and must follow precise organization that complies with stringent research protocols to ensure the safety of the participants.
Clinical research, or the requirement for scientific rigor
Clinical research designates all scientific studies carried out on volunteers in order to develop medicines and medical devices.1 Clinical trials are usually conducted in a hospital setting or a doctor’s office, under the supervision and responsibility of physicians.
Clinical trials are used to assess the safety, efficacy, and use of treatment in humans so that a new medicine can be made available on the market. They require the application of a rigorous scientific method in order to guarantee both the therapeutic benefit of the proven solution and the safety of the participants. In addition to its therapeutic interest, clinical research also contributes to the development of medical and biological knowledge.2
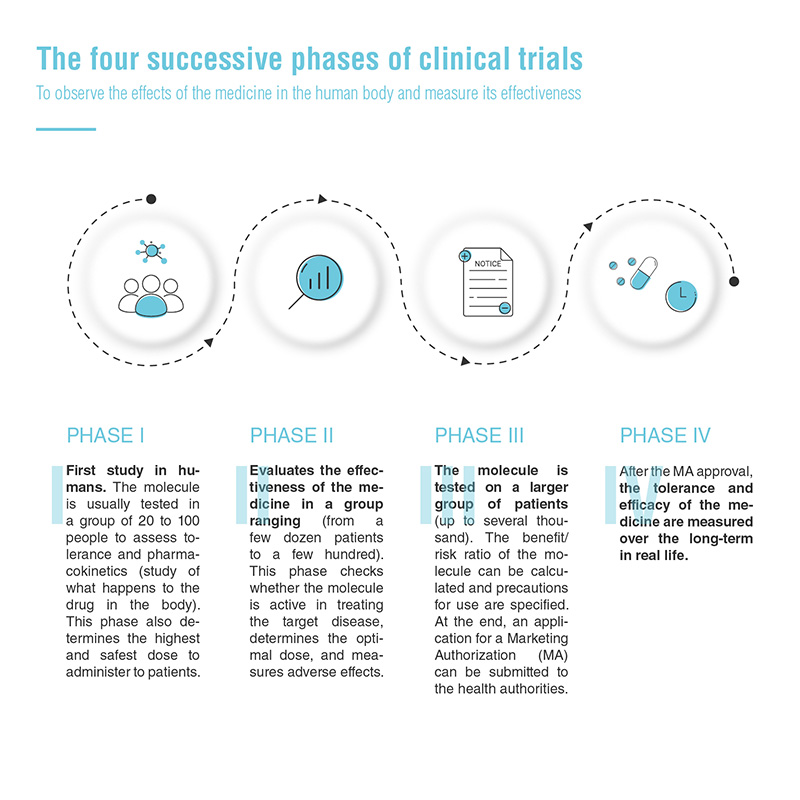
Four phases of clinical trials
Pre-clinical trial validation is a prerequisite for starting clinical trials. This involves testing the molecular entity on animals before administering the candidate drug to humans.
The development of a new medicinal product typically consists of four successive phases of clinical trials, which aim to observe the effects of the drug in the human body and measure its effectiveness.
Currently, only one in ten medicines passes all the stages.3 However, for oncology or a rare disease, the clinical development process of a drug can be accelerated: there can be fewer patients and certain phases of development may be combined.
Pharmaceutical companies, researchers, doctors, and patients are all mobilized!
Clinical trials generally take several years, sometimes up to ten years, involving the daily commitment and efforts of numerous stakeholders in order to find new therapeutic solutions that are vital for treating patients.
Pharmaceutical companies most often initiate clinical trials, as well as their funding. All clinical trials are carried out by qualified health care professionals, called investigators. They are in charge of proper trial conduct, and are responsible for the inclusion of patients in the study and for their safety. As for the participants, they are always volunteers.
Ensuring transparency of results within a stringent legal framework
To start a clinical trial, various authorizations are required from the relevant authorities of each country. Approval times vary from country to country (from 30 days to more than 200 days).
Each participant must receive all information relating to the study in writing (including benefits and risks) and must give their consent by signing an informed consent form before they can be selected. The Nuremberg Code and the Declaration of Helsinki of 1964, as well as their successive updates, advocate the ethical aspects of medical research worldwide.
The publication of the results of a clinical trial is of major interest to medical and scientific research because it helps to ensure the transparency of results, whether positive or negative. The Declaration of Helsinki stipulates, “Every research study involving human subjects must be registered in a publicly accessible database before recruitment of the first subject.”

NEW CHALLENGES AHEAD

AND SERVIER?
The Group’s clinical trials are managed by teams with a high level of expertise. The proper conduct of these studies requires a good organization involving the teams in charge of international coordination and locally in 15 clinical development centers divided into 3 hubs, which thus enable clinical studies to be managed worldwide.
Servier publishes 100% of the results of its European clinical trials on the European Union Clinical Trials Register website.
Servier is committed to involving patients, directly or through patient associations, to develop medicinal products that best meet their needs. These activities include clinical trial protocols, the development of consent forms and patient awareness brochures, patient feedback following a study, and adapted summaries of study results.
Digital technology and data increase agility, efficiency and productivity to speed up the research process, the iterative molecule identification procedure and clinical trials, while also developing a more detailed understanding of patients. SCORE, the program that digitizes clinical trials, falls within this ambition as it encourages remote dialogue between patients and medical and scientific teams by enabling them to access data in real time, therefore making it easier to make decisions. The FEDERATES platform improves the communication of information and data in real time and accelerate the various research stages.
1 https://clinicaltrials.servier.com/about-clinical-trials/
2 https://www.inserm.fr/recherche-inserm/recherche-clinique
3 https://www.leem.org/recherche-et-developpement
4 https://www.clinicaltrials.gov/ct2/help/glossary/phase
5 Goldacre Ben, DeVito Nicholas J, Heneghan Carl, Irving Francis, Bacon Seb, Fleminger Jessica et al. Compliance with requirement to report results on the EU Clinical Trials Register: cohort study and web resource BMJ 2018; 362 :k3218
