Launched in 2018, PROMETCO is the first real-world study conducted by Servier in oncology, in metastatic colorectal cancer (mCRC). A look back at the initiative, its first achievements and upcoming results.
At Servier, patients are at the heart of every decision. This ambition is symbolized by PROMETCO, the first real-world study (see box) in oncology – and more specifically in metastatic colorectal cancer – conducted by Servier teams.
Developed in collaboration with the patient association Digestive Cancers Europe (DiCE), the PROMETCO real-world study will enable the Group to form a large cohort of patients, expressing the reality of metastatic colorectal cancer management from the point of view of the patient and the physician.

First results…
In June 2021, initial results on the demographics and clinical characteristics of the first 277 patients enrolled in the study were presented at the European Society for Medical Oncology’s World Congress on Gastrointestinal Cancer (WCGIC) 2021. Differences in routine molecular testing practices across countries were revealed.
In January 2022, at the ASCO-GI (American Society of Clinical Oncology) conference on gastrointestinal cancer, Servier teams presented a poster on the subject. This poster presented the first data obtained from interim analyses and highlighted the heterogeneity and the complexity to care patients with metastatic colorectal cancer.
In addition, the PROMETCO teams published on a dedicated website an interactive visual of these first results, for the PROMETCO community of investigators.
… and publications
A publication in the scientific journal Future Onclogy describes PROMETCO as the first international real-world study to investigate the continuum of care for patients with mCRC. The article notes that the project will provide valuable information to the gastrointestinal medical community.
This publication was relayed digitally, using a variety of current communication media: podcast and poster.
The patient association Digestive Cancers Europe also published a communication underlining the involvement of patients in this study and the interest of this long-term collaboration.
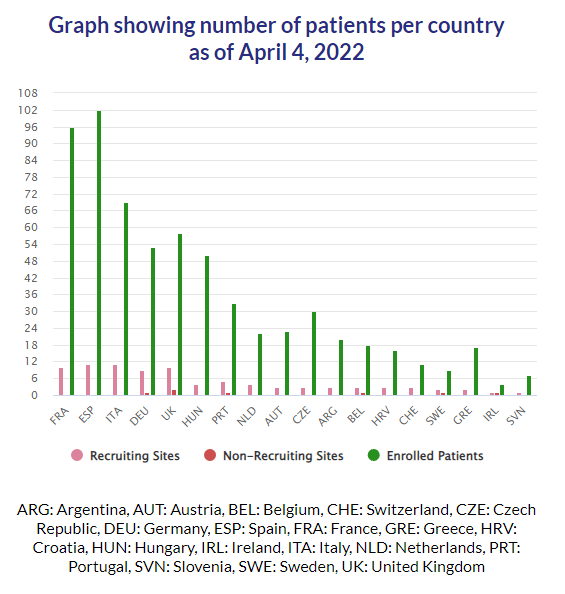
PROMETCO in figures:
1,000 patients: recruitment target
125 research sites involved
18 countries involved
The next steps
The year 2022 marks the final stretch of patient recruitment for the PROMETCO study. Thanks to the ongoing efforts of the various research sites, more than half of the 1,000 patient recruitment target has already been reached.
A publication co-authored with patients highlighting the involvement of patients in real-world studies and what they can gain from them, as well as an abstract for ESMO, are also planned for 2022.
Finally, the study report will be published by 2023, with the hope of improving future practices, particularly on the performance and personalization of treatments.
Understanding real-world studies
Improving medical knowledge
Real-world data are collected from a variety of sources and relate to the lived reality of patients and doctors, drawing on information such as the delivery of health care, treatments, as well as the impact of the disease and its treatment on patients’ lives. In other words, these data reflect the real lives of people affected by the illness in question and complement those derived from traditional clinical trials. Such additional robust analysis on the experience people have of living with diseases and medical treatment helps to expand therapeutic understanding.
A structured methodology for robust findings from clinical trials
It is important to first understand the needs of all stakeholders in order to precisely identify the matters that require research, whether in relation to medical concerns, medical economics, or even regulation. Although it takes time to identify sources of data and deploy a suitable methodology, such steps are essential to obtain robust findings. Data are then analyzed using increasingly high performance digital platforms that enable the Group to compile ever larger data bases so as to exploit ever more homogeneous and standardized data.
