Innovation, boldness, collaboration: what does the daily life of a startup really look like? The Insights teams delved into the world of the biotech company SparingVision, whose laboratories are hosted by Spartners by Servier & Biolabs, the startup accelerator affiliated with the Paris-Saclay Servier R&D Institute. Discover behind the scenes of this successful biotech company!
Who is behind SparingVision and what is their mission?
SparingVision is a biotech company specializing in the development of genomic medicines for treating retinal diseases that lead to blindness, such as retinitis pigmentosa and certain forms of age-related macular degeneration (AMD). To this day, these pathologies remain without a lasting treatment.
Founded in 2016 by Prof. José-Alain Sahel and Dr. Thierry Léveillard, SparingVision’s mission is to innovate in gene therapy and genome editing of the genome to combat blindness.
Learn more: sparingvision.com
Today, SparingVision is made up of more than thirty employees, all dedicated to developing therapeutic solutions for patients with retinal diseases. We followed several teams – the R&D based at Spartners, the quality team, as well as the support services located in Paris – to understand how each of them contributes to the therapeutic innovation at SparingVision, and the key moments that shape the daily life of the startup.
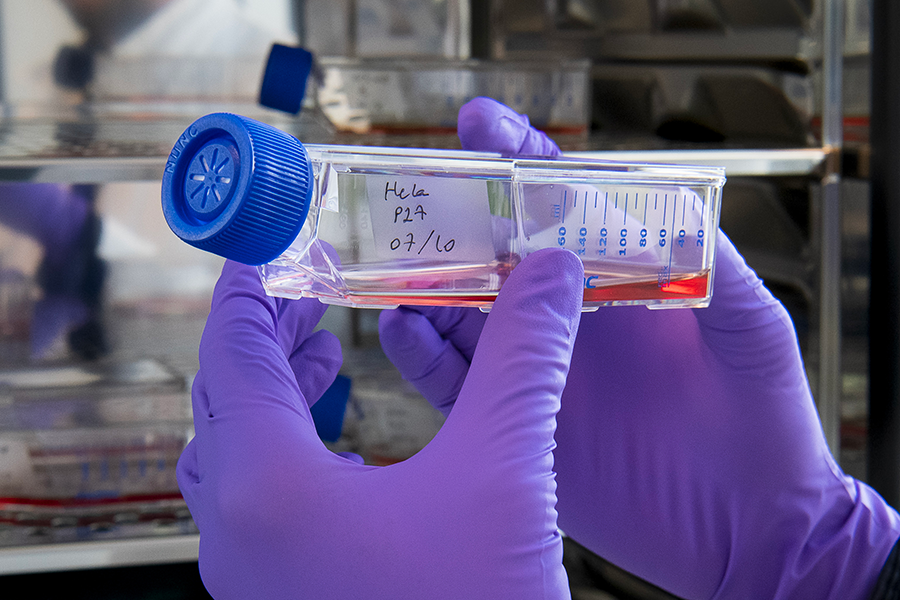
Picture 1 / Identify most effective drug candidates
First, we met Benoît Gachet, the first link in SparingVision’s value chain. He’s an R&D engineer, specializing in cell and molecular biology. His role is crucial: he studies the pharmacology of drug candidates, particularly their efficacy in vitro and in vivo. The tissues are analyzed in the laboratory. The findings of these studies allow us to select the most promising treatment candidates for the remainder of the clinical development.
“I develop molecular biology techniques to assess the efficacy of drug candidates. These studies allow us to identify the most promising treatments for future clinical trials.”
Benoît Gachet, R&D engineer, specializing in cell and molecular biology


©Corinne Simon
Benoit checks the efficacy of the drug in the retinas of animals treated with the gene therapy. Using fluorescent labelling techniques, he visualizes the effects of the drug candidate on the target cells

The safety of the drug candidates is evaluated in partner laboratories, specializing in drug safety regulatory studies.
Picture 2 / Production and Quality control of drug Candidates for clinical trials
The drug batches selected for the clinical development are produced by partner subcontractors (CDMOs – Contract Development and Manufacturing Organizations), specializing in the production of gene therapies, under the supervision of SparingVision’s Drug Candidate Production and Control (Chemistry, Manufacturing and Control (CMC)).
The batches are then tested by the quality control laboratories of these CDMOs, or other subcontractors. Fabien Dorange, Director of Analytical Sciences at SparingVision, oversees the development of analytical methods: he supervises the planning of analyses and monitors the results. The tests performed verify the drug’s concentration, biological activity, purity, microbiological and particulate quality, as well as other standard controls (such as appearance, pH, etc.). This series of physicochemical and biological tests on each drug batch is crucial to ensure compliance.
SparingVision develops analytical methods, specific to its products, enabling a deeper understanding of them and allowing these methods to be transferred to subcontractors for application during later clinical phases. In the development of these methods, we find Baptiste Erout, an engineer within the Drug Candidate Production and Control team. His work involves, among other tasks, demonstrating and quantifying the biological activity of the proteins produced.

©Corinne Simon
Baptiste analyzes the functionality of batches on cells in vitro.

“All these steps are essential to ensure the success of a drug candidate. They allow us to establish the foundation for a clinical trial and define evaluation criteria we aim to measure.”
Fabien Dorange, Director of Analytical Sciences at SparingVision
“The quality assurance department is responsible for establishing a quality system, “toolbox”, to carry out manufacturing operations in compliance with best practices. This ensures the reproducibility of processes, the quality of the drug, and patient safety. Quality is implemented concretely on the ground and is everyone’s responsibility. It is based on two key principles: continuous improvement and risk management.”
Gaëlle Fanon, Quality Assurance Manager at SparingVision

SparingVision’s approach: Genotype-Independent Gene Therapy
Gene therapy is an innovative medical strategy that uses DNA as a drug. The goal is to address a mutation (a variation in the patient’s DNA) that causes the disease, by introducing therapeutic DNA into the affected cells. Some diseases, such as retinitis pigmentosa, can be caused by a large number of pathogenic mutations identified to date in over 80 different genes. In SparingVision’s “genotype-independent” approach, the DNA introduced is not intended to replace the defective gene, but to induce retinal cells to produce a protein required for proper photoreceptor function, regardless of the gene responsible for the disease. SPVN06, SparingVision’s most advanced drug candidate, could thus potentially address all patients suffering from retinitis pigmentosa.
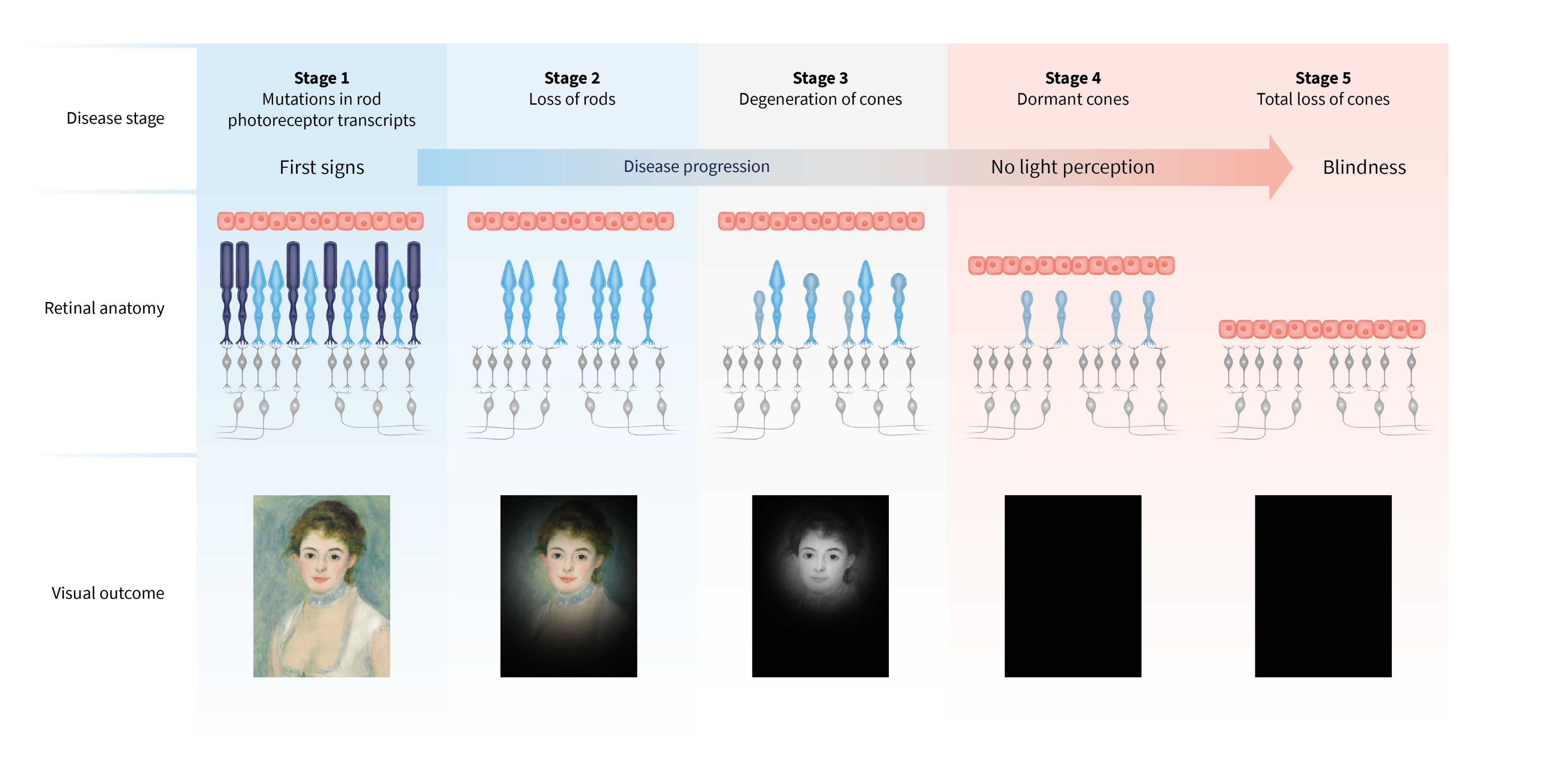
Retinitis pigmentosa is genetic eye disease that affects over 1.5 million people worldwide.1 It initially impacts night vision and then progresses to impair daytime vision. In advanced stages of the disease, patients develop “tunnel vision”, where they lose the ability to perceive movement or objects in their peripheral field of vision. However, they may still retain central vision, allowing them to perceive their surroundings. Some patients also lose this valuable central vision as well, leading to complete blindness. SparingVision targets this disease with two main objectives: to slow or stop vision loss, and/or to restore vision for blind patients, regardless of the gene responsible for the disease.
Learn how retinitis pigmentosa is characterized at different stages of disease outcome.
Picture 3 / Interaction with Biolabs and Servier teams
“One of Spartners’ advantages is its proximity to experienced scientists in various fields and techniques. We can also use Servier’s technological platforms when specific equipment is needed.”
Benoît Gachet, R&D engineer, specializing in cell and molecular biology


Picture 4 / Gaining Recognition at International Scientific Conferences
SparingVision regularly participates in international conferences where teams present their scientific and clinical results. Beyond sharing knowledge, these events are crucial for establishing collaborations with other researchers, pharmaceutical companies, and potential investors.
Picture 5 / Celebrating Success Together
In 2023, SparingVision initiated phase 1/2 of clinical development of SPVN06, a drug candidate for retinitis pigmentosa. This milestone marks significant progress and highlights confidence in the biotech’s work. All teams are fully committed to ensuring the success of this study and are already planning the next steps. The objective is to evaluate the safety and tolerability of the drug candidate during phase 1/2 before moving to phase 3, where the therapeutic benefits will be tested on a larger group of patients.


SparingVision’s team. From a biotech startup with just four people in 2016, SparingVision has grown to close to 40 employees! SparingVision is now in the clinical phase for its most advanced drug candidate targeting retinitis pigmentosa.
[1] Verbakel SK, van Huet RAC, Boon CJF, et al. Non-syndromic retinitis pigmentosa (Retinitis Pigmentosa: Burden of Disease and Current Unmet Needs – PMC (nih.gov)) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9232096/
