Counterfeit medicines: What are they?
Counterfeit pharmaceutical products can take many forms. For example, they may contain the correct drug substance in the wrong dosage, another active compound, or even no active component at all. They may also be genuine medicines that have been diverted for illegal resale, either because they were improperly stored or because they are past their expiration date. Often, patients are unaware that they have purchased a counterfeit product. In other cases, falsified medicines are intentionally sought out for use as doping or psychotropic agents.

Key figures
In 2016, global trade in counterfeit pharmaceuticals reached 4.4 billion USD.1
In 2021, counterfeit medicines accounted for a quarter of all counterfeit goods seized by European customs in postal traffic.2
10% of medicines distributed in developing countries are substandard or counterfeit.3
Counterfeit medicines: a fast-growing threat
The market for illicit pharmaceutical products is booming. Until a few years ago, falsification mainly involved comfort drugs – erectile dysfunction treatments, for example, were the most common type of counterfeit product for many years – but now all types of drugs and therapeutic areas are affected. From antimalarials to cancer therapies, and from treatments for chronic illnesses to anxiolytics, no product is spared in today’s market. These counterfeit products are both manufactured and distributed worldwide.
This surge is due to a variety of factors, beginning with the explosion of e-commerce, which has led to the proliferation of thousands of unregulated sites. Added to this is the emergence of a self-medication and self-diagnosis culture in countries where access to medical consultation has become too complex or complicated. Whereas patients were previously reluctant to consume medicines without medical advice, some are now being enticed by the idea of purchasing medicines without a prescription, particularly online.
At the same time, the market for counterfeit medicines is becoming highly professionalized. Counterfeiters no longer hesitate to target high value-added products, demonstrating the same degree of agility as pharmaceutical industry players, as well as a remarkable ability to adapt to patient needs and societal trends. This agility was particularly evident at the outset of the Covid-19 pandemic, when counterfeiters were swiftly able to market much needed equipment such as masks, sanitizing solutions, and oxygen kits. To counteract the growing number of illicit products on offer, pharmaceutical industry stakeholders – in cooperation with law enforcement authorities – have put necessary measures in place. Another factor signaling the need for action is the increasing use of parcel post to transport falsified medicines, which exploits the explosive growth in the number of parcels in transit every day.
7 million dollars4
This is the value of pharmaceutical products seized during Operation Pangea XVI, a large-scale Interpol-driven operation against counterfeit medicines carried out in October 2023. It led to the closure of more than 1,300 fraudulent websites marketing erectile dysfunction treatments, psychotherapeutic agents (including anxiolytics and antidepressants), sex hormones, and medicines for gastrointestinal disorders.
Counterfeit pharmaceuticals are now a highly lucrative business. According to Europol, it is 10 times more profitable than heroin trafficking. Furthermore, it is considerably less risky than narcotics trafficking because the penalties are not sufficiently deterrent and are not uniform at European and international levels. In France, for example, prison sentences are limited to 7 years, compared with 30 years for narcotics trafficking.5
How can we protect patients from counterfeit medicines?
Faced with the burgeoning market for counterfeit medicines, law enforcement authorities, health authorities, and the pharmaceutical industry are joining forces to dismantle criminal networks and ensure patient safety. Pharmaceutical companies are also actively involved in the development of innovative ways to improve product traceability and protect patients. One such initiative is pharmaceutical supply chain aggregation, a system that tracks a medicinal product from one level of packaging to the next. In other words, before a box of medicine moves on to the next stage in the supply chain, tracking data is aggregated in a database, starting from the individual unit (lowest level) up to the pack, case, and pallet (highest level).
Cooperation, a vital factor in the war on counterfeiting
Cooperation among all stakeholders is crucial to countering the growth of the illicit pharmaceuticals market.
Each player thus has a specific role to play, essential to the implementation of a comprehensive anti-falsification strategy. Pharmaceutical companies, for example, are responsible for global surveillance, enabling them to identify criminal networks. They are therefore in a position to raise the alarm, both to their counterparts (private-private partnership) and to law enforcement authorities (public-private partnership), who can then work together to uncover and take down criminal networks and punish traffickers (public-public partnership).
As part of the G5 santé initiative, the first French public-private partnership was initiated by Servier in cooperation with the Central Office for Coordinating Environmental and Public Health Crime (Office Central de Lutte contre les Atteintes à l’Environnement et à la Santé Publique – OCLAESP). The project was designed to combat the sale of illicit products marketed during the covid-19 pandemic and has since been extended to all illicit products.
“Given the danger that illicit pharmaceuticals pose to patients, no one is completely indifferent to the subject of counterfeiting. We can therefore count on unlimited collaboration between all involved.”
Meriem Loudiyi, Director of Trademarks and Anti-Falsification Department, Servier
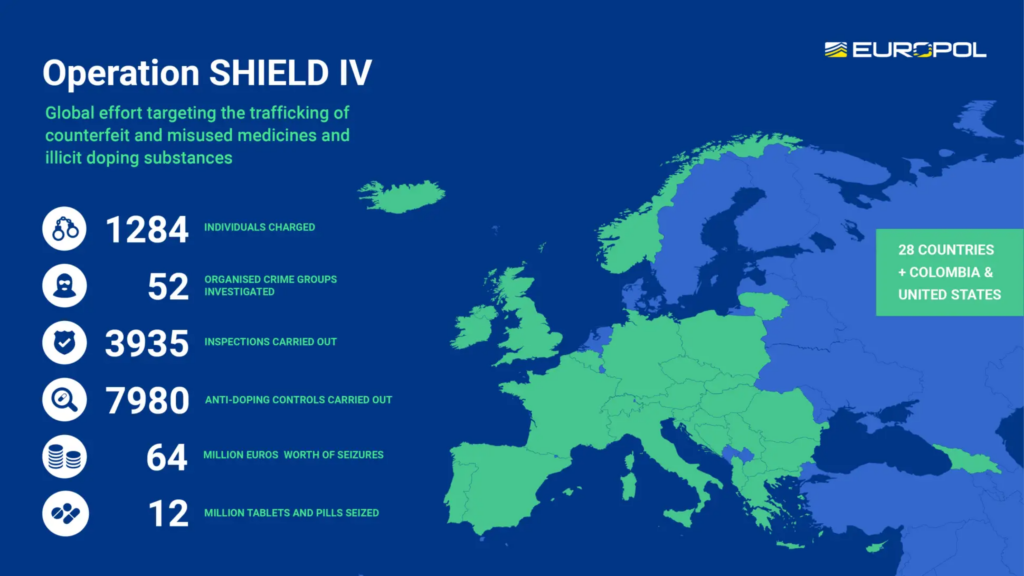
SHIELD6, a Europe-wide network to combat pharmaceutical trafficking
Mindful of the importance of cooperation in curbing the proliferation of counterfeit medicines, the European Union created SHIELD in 2020. This anti-trafficking network is currently managed by Europol. It brings together key players including OCLAESP, the European Anti-Fraud Office, Frontex, the European Union Intellectual Property Office, and the World Anti-Doping Agency.
Operation SHIELD IV demonstrated the remarkable power of stakeholder collaboration in the anti-counterfeiting movement. Rolled out in 30 countries across three continents7 between April and October 2023, it united public and private forces, including law enforcement, judicial authorities, customs authorities, and medical agencies. Pharmaceutical companies — such as Servier — also played a major role, sharing key data from the market surveillance they carried out throughout the year.
This SHIELD operation resulted in the seizure of illicit medicines and healthcare products worth 64 million euros.
Technological innovation for the protection of patients
Serialization – a mandatory measure in Europe – plays an essential role in the ongoing effort to combat pharmaceutical counterfeiting. It involves the use of a unique identifier on each box of a prescription medicine to identify the product. This identifying feature is affixed by the pharmaceutical manufacturer and scanned by the pharmacy before the drug is dispensed. A tamper-proof seal must also be present on all medicinal products, to confirm that the container has never been opened.
To protect the integrity and quality of their medicines, some pharmaceutical companies, including Servier, have chosen to go beyond the obligation of serialization by setting up a system of supply chain aggregation. This innovation requires that the entire container (box or carton) in which the medicines are shipped to pharmacies be specifically coded or marked. It therefore offers an additional level of protection.
More generally, new developments in digital tools are constantly opening up new opportunities to improve product traceability. In France, for example, a trial aimed at replacing medicine leaflets with digital leaflets that can be accessed by scanning a QR Code was launched in 2024. By introducing a digital dimension, these QR Codes make falsification more complex. A similar project will also be launched in Brazil.
For patients, the watchword is vigilance
Despite the efforts of law enforcement, health authorities, and the pharmaceutical industry to protect patients, ongoing vigilance is required when purchasing pharmaceutical products. In addition to always purchasing medicines from a licensed pharmacy or a certified online pharmacy, patients must pay close attention to the condition of the packaging, the manufacturing and expiration dates, and the quality of the package leaflet.
Listen to the first-hand account from the Jan Geissler, co-founder of the CML Advocates Network and patient representative in various committees, about a community perspective and experience with counterfeit drugs.

[1] OCDE – 2020
[2] European Commission / EUIPO—2021
[3] OMS – 2017
[4] Interpol – https://www.interpol.int/fr/Actualites-et-evenements/Actualites/2023/Une-operation-d-INTERPOL-cible-les-medicaments-illicites-dans-le-monde
[5] Gendinfo – https://www.gendarmerie.interieur.gouv.fr/gendinfo/actualites/2022/shield-un-bouclier-de-protection-contre-le-trafic-de-medicaments
[6] https://www.gendarmerie.interieur.gouv.fr/gendinfo/actualites/2022/shield-un-bouclier-de-protection
[7] Europol – https://www.europol.europa.eu/media-press/newsroom/news/fake-medicines-worth-eur-64-million-eu-markets
