Translational medicine is a significant component of clinical research and development. It is a cross-disciplinary field involving researchers, clinicians and patients in an effort to transition innovations from the research to the human clinical development phase and to enrich fundamental research with observations from clinical research.
Insights from Fabien Schmidlin, Director of the Translational Medicine department at Servier.
Translational medicine lies at the interface between basic research, with the quest to understand the mechanisms of disease development, and clinical research which aims to assess the efficiency and safety of new treatments for patients.
This collaborative discipline is essential to translating scientific discoveries into new treatments that improve patient health. Translational medicine combines the skills of researchers, pharmacologists, and clinicians to evaluate new treatments and ultimately accelerate all the stages of drug development. It is applicable to all diseases.
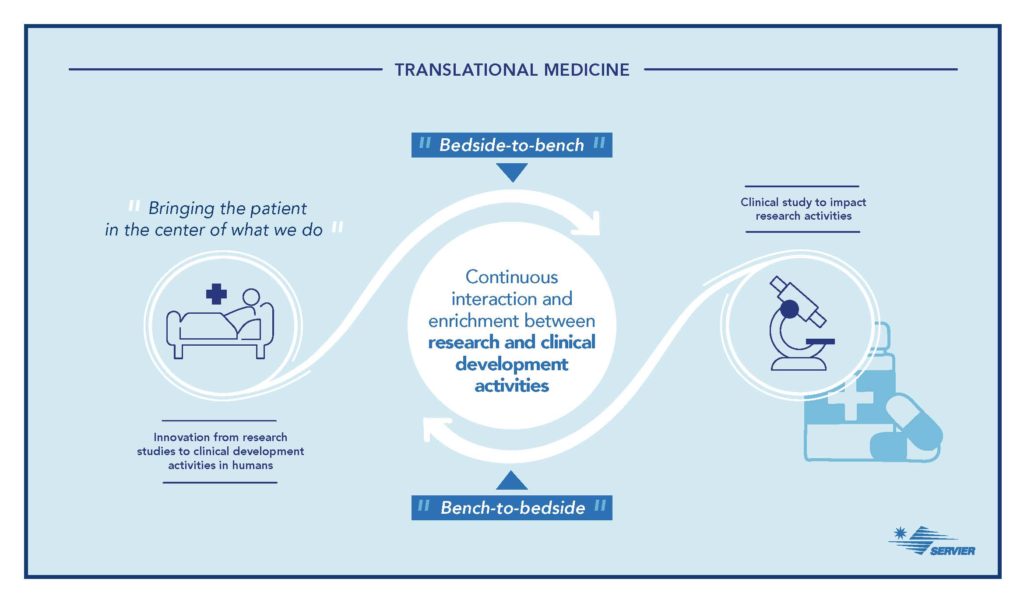
Starting from clinical observations and patient medical needs, “Bedside-to-Bench,” translational medicine promotes the search for new molecules by accelerating their evaluation in patients, “Bench-to-Bedside.”
Patient-Benefitting Goals
According to Fabien Schmidlin, “Previously, a significant gap existed between the worlds of laboratory research and clinical drug development. Today, translational medicine more closely connects research and care, which ultimately accelerates decision-making for the launch of a drug on the market.”
“Previously, we tried to treat all cancers with a single molecule,” emphasizes Fabien Schmidlin. “Today, we are trying to identify very early on which patient subpopulations will respond effectively to treatment. This is called personalized medicine. Today we have targeted therapies for some cancers with a response rate of nearly 90%.”
Translational Medicine at Servier
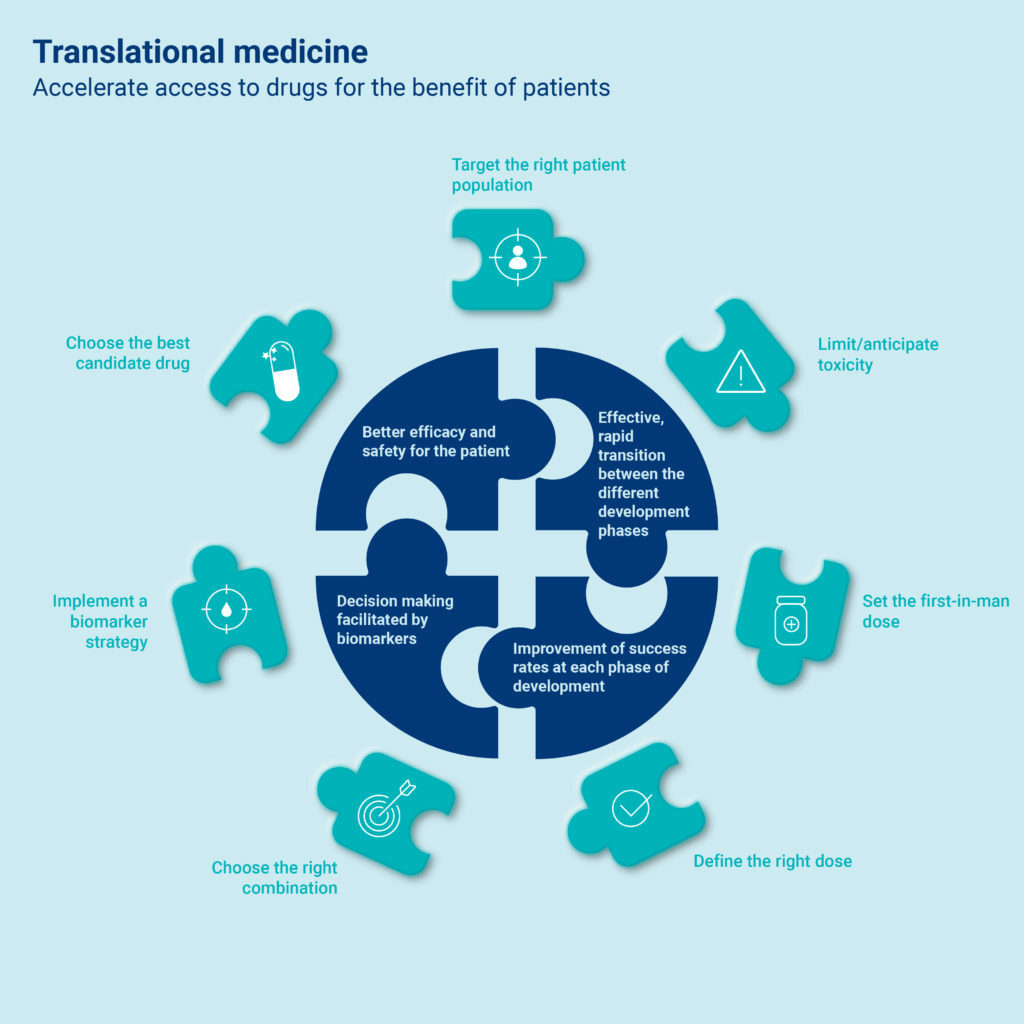
In 2020, Servier created a department dedicated to translational medicine.
Its goal is to support research by facilitating an efficient and rapid transition of the Group’s portfolio of molecules from the preclinical phase (in which a molecule is evaluated in vitro in cell culture and in vivo in animals) to the FIM phase (“first in man”: first dose given to humans), which is the first step in clinical trials.
This department, in close collaboration with other R&D departments, accelerates the new drug development process and thereby speeds market authorization to serve patient.
“The patient is at the heart of the process and plays a fundamental role in the discovery of new treatments,” explains Fabien Schmidlin. “Patient involvement allows the collection of biological samples and associated clinical data that are essential to identify and to offer new therapeutic solutions.”
At the heart of translational medicine: biomarkers
What is their role in drug development?
Biomarkers are essential to the drug development process. They increasingly facilitate decision-making at each stage of development. Biomarkers make possible to monitor and predict the efficacy and safety of products during development and to select patients who are likely to respond.
The development of biomarkers is crucial to translational medicine programs. There are different types for various purposes:

AND SERVIER?
Servier is committed to an innovative and patient safety-centered strategy in performing clinical studies with biomarkers. This biomarker-driven strategy should strengthen the development of targeted therapies for the benefit of patients.
Currently, most of the biomarkers in development by Servier are in oncology. However, the search for new biomarkers offers new perspectives in the development of molecules for autoimmune diseases (lupus, for example) and neurological diseases.
The newly created Biomarker Development department at Servier aims to truly accelerate personalized medicine to improve the management of therapeutic care and patients’ quality of life.
